The purpose of the dose-response study was to find out the most effective dose of TBL for treating SAD symptoms. 89 patients suffering from severe SAD were randomly distributed in one of three treatment groups. One group received a low (1 lumen, 2400 lux), a medium (4 lumen, 10000 lux), or a high dose (9 lumen, 21500 lux) of bright light treatment. The test lasted four weeks. The patients received TBL through their ear canals for 12 minutes every morning.
At the beginning, during the test period and at the end, the symptoms of depression and cognitive performance of the patients in all three groups were recorded using standard psychiatric instruments such as the Beck Depression Inventory (BDI) and the Trail Making Test (TMT).
The results were interesting in two ways: On one hand, according to the BDI, they showed a significant reduction, at least 50%, of the symptoms of depression on 74-79% of the patients in all three groups. There was also a significant improvement of patients' cognitive performance compared to baseline. And the patients in all three groups reported feeling better after the four-week trial. On the other hand, there was no difference in the dose-response effects between the three different intensities of TBL. When designing the study, the low-intensity group was created as a placebo group with a dosing below the saturation threshold, i.e. too low to produce an effect in the patients. Based on the results, this theory had to be dismissed, as the patients in all three groups became better.
Could this mean that the entire effect could be solely due to placebo? The lack of a true placebo condition for light interventions is the biggest challenge when it comes to study the effects of visible bright light therapy.
Meta-analysis in earlier studies on pharmacological therapy in major depression showed an effect of 29.7% for placebo, which accounted for 68% of the effect in the treatment group. Since Valkee is not a pill but a medical device with transcranial mechanism, it makes sense to compare the results also to the placebo effect of a similar technique such as repetitive transcranial magnetic stimulation (rTMS). In major depression, this non-pharmacological treatment has shown a lower placebo response than pharmacological therapy. Thus, it is not likely that the explanation for the alleviation of the symptoms found in the Valkee study lies entirely in the placebo effect.
Wellnessproducts > Light therapy > Valkee research
Study, Published In BMC Psychiatry: Even a Small Dosage of Bright Light via Ear Canal Alleviates SAD SymptomsTwo aspects are at the heart of the Valkee research: to understand the working mechanism of transcranial bright light (TBL), and to determine its clinical effectiveness on people. A research paper on the mechanism of TBL and melatonin suppression has already been published in the journal Chronobiology International. Now, the results of a TBL dose response study in treating patients suffering from symptoms of seasonal affective disorder (SAD) has also been published in the peer-reviewed US journal BMC Psychiatry.
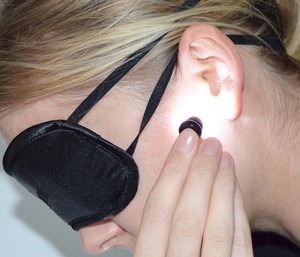 Published on 17.09. by Thomas Toernell  Light therapy Light therapyDo rainy days or the dark winter months leave you feeling a bit gloomy? Try using a light therapy machine to add some natural light to your life - revitalizing and reenergizing your mood.  Charger for Valkee light therapy device, CHF 19.00 Charger for Valkee light therapy device, CHF 19.00Charging via power outlet: For Valkee 1 & Valkee 2 |
- FREE DELIVERY (ECONOMY)
- Safe shopping: Payment with invoice, credit card, Paypal or Postfinance
- Warehouse in Switzerland (Widnau SG)


 Extra Headset for Valkee2, CHF 59.00
Extra Headset for Valkee2, CHF 59.00